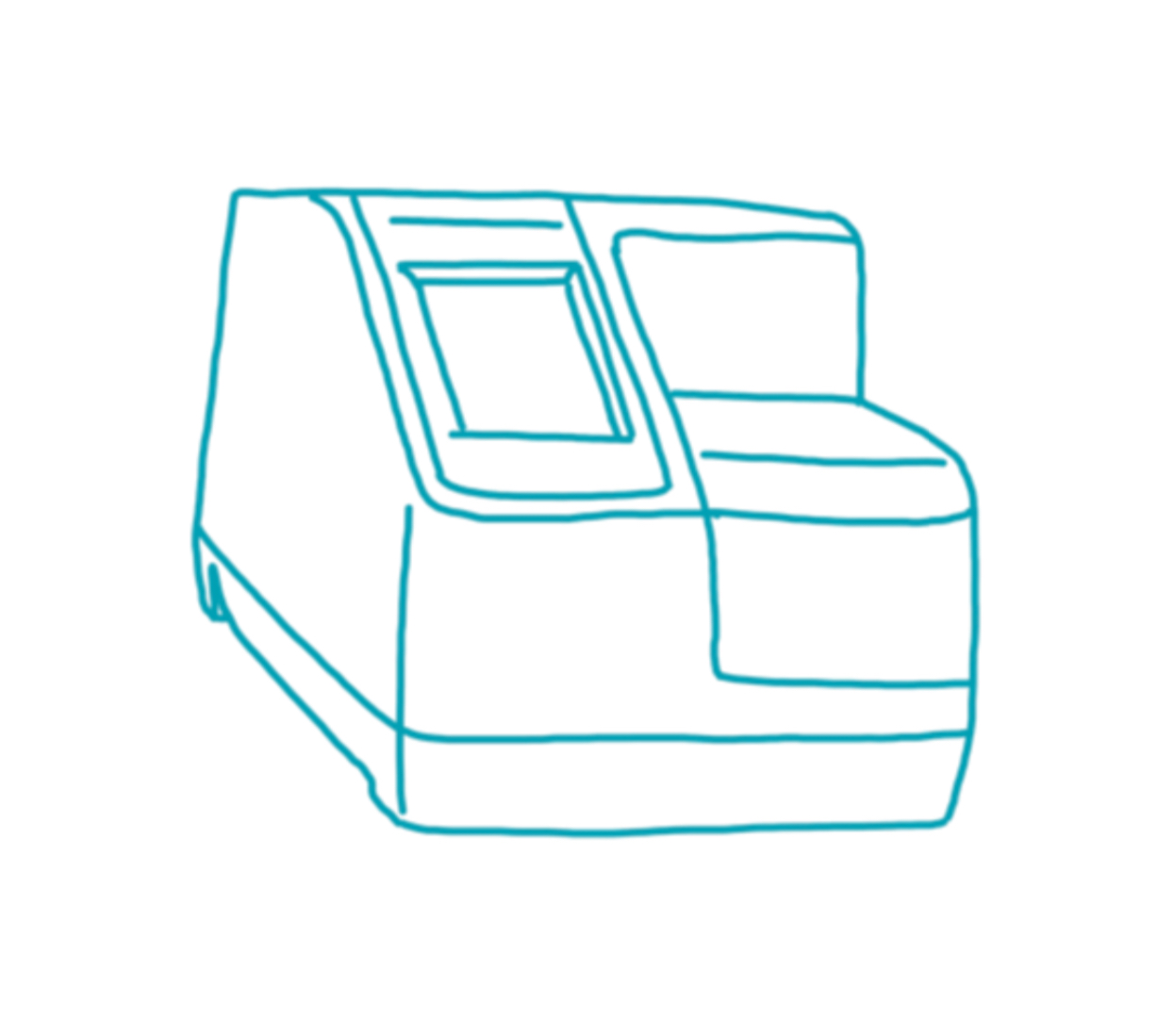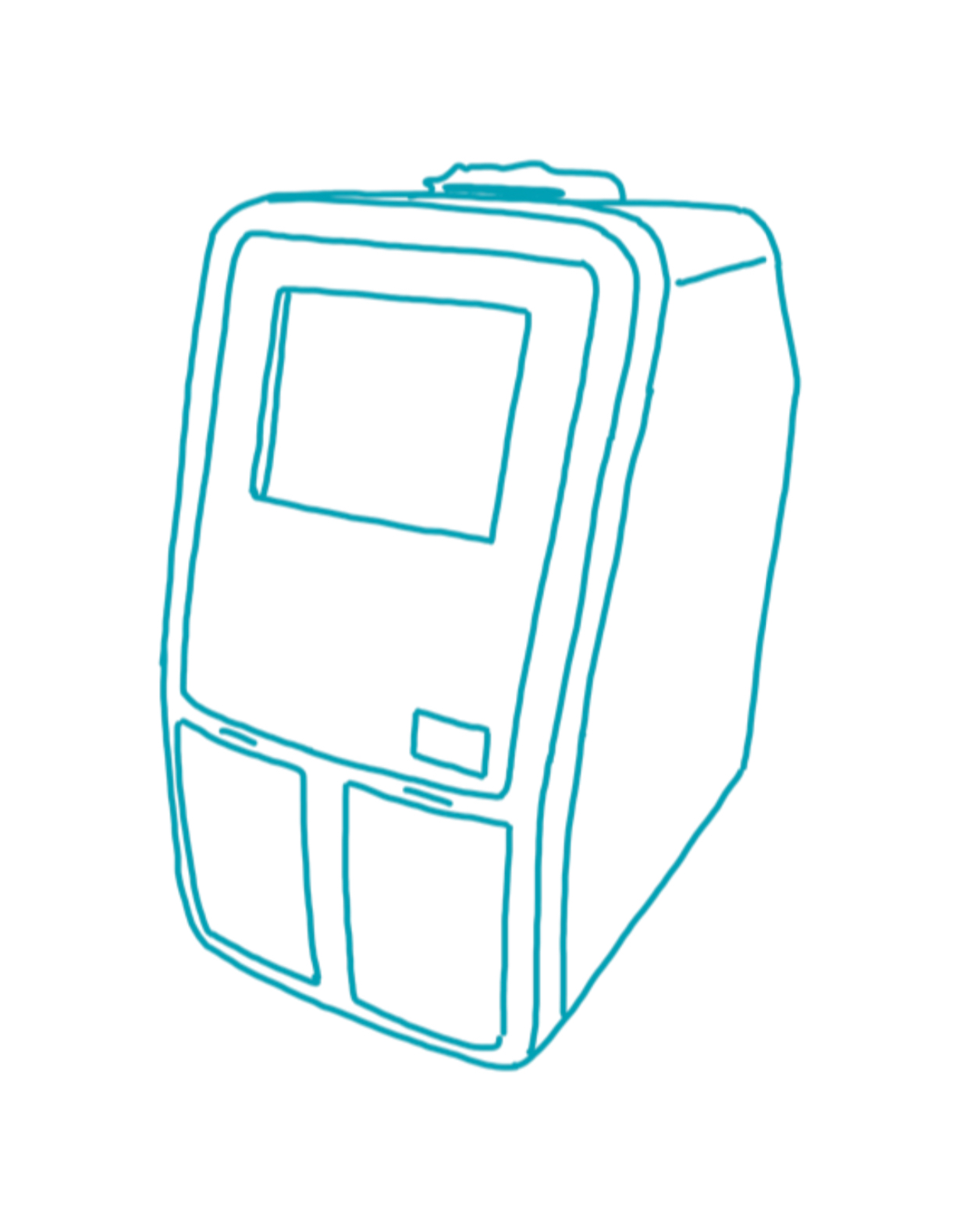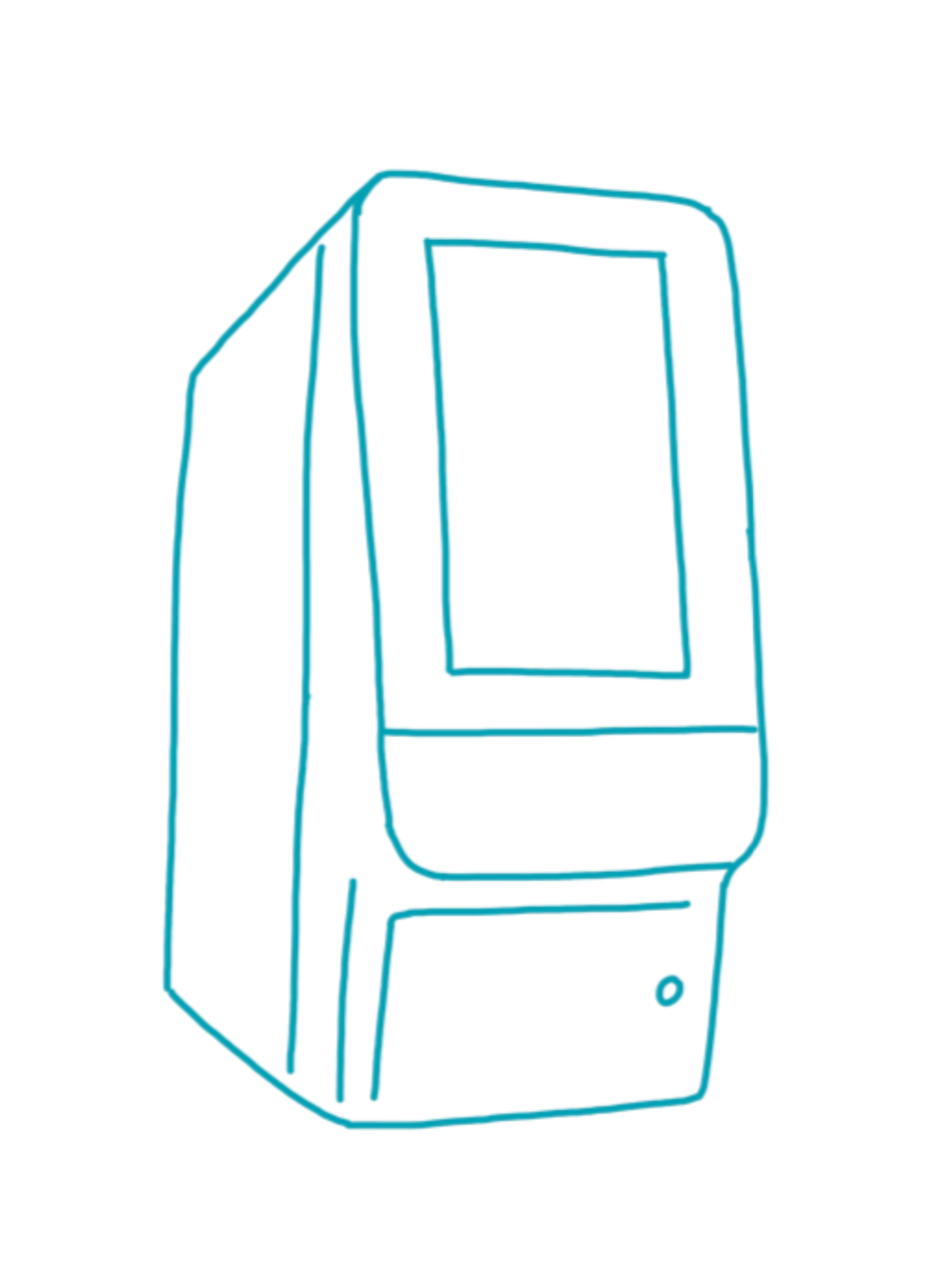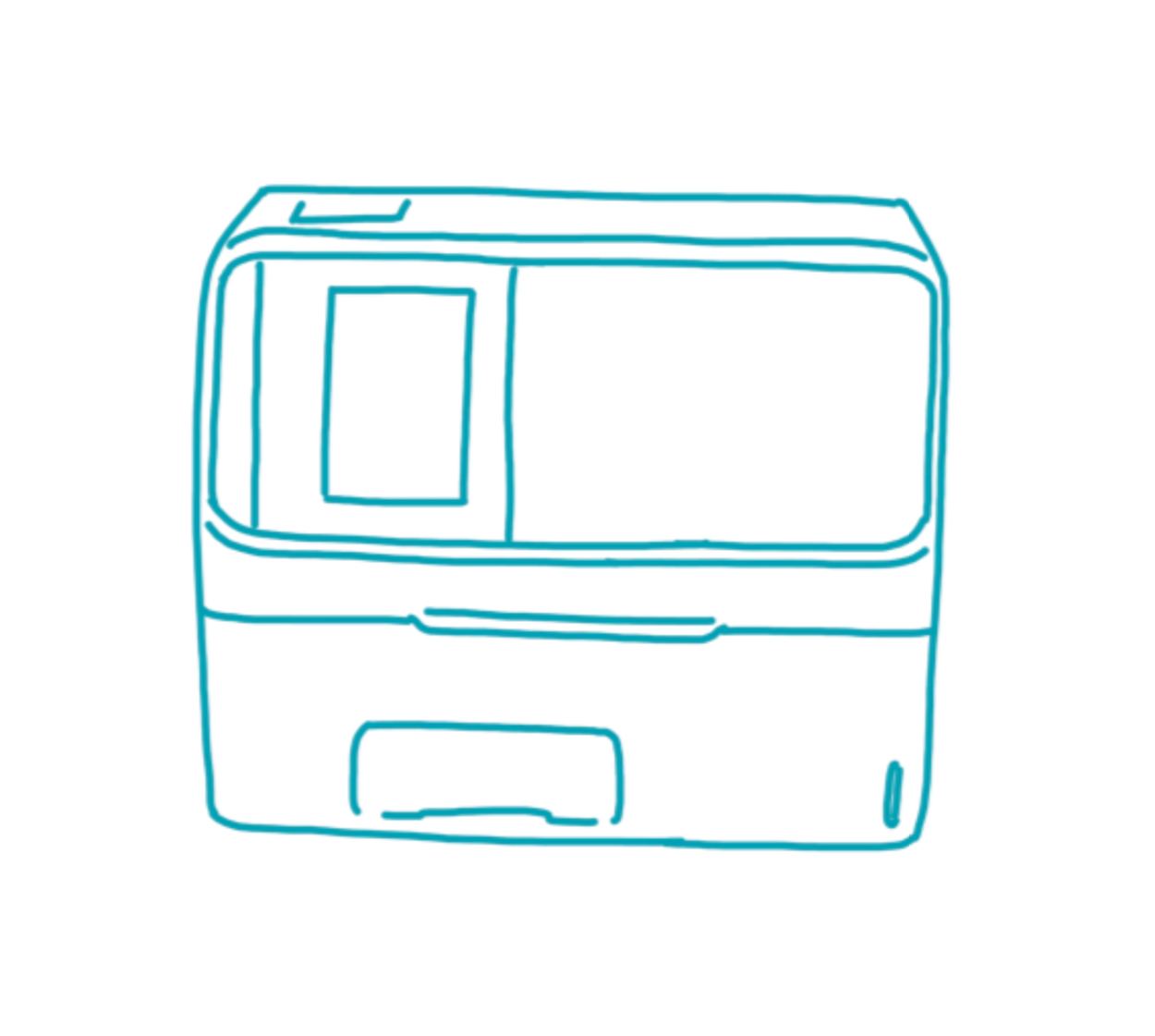
Pointcare cM4
Manufacturer: MNCHIP
Weight: 2.5 kg
Dimensions: 12.5 × 21 × 17.5 cm (B × T × H)
Connectivity: LAN , WLAN , Bluetooth
Parameter overview
Alanin-Aminotransferase
Albumin
Alkalische Phosphatase
Amylase
Aspartat-Aminotransferase
Bilirubin
Alanin-Aminotransferase
Amylase
Bilirubin (direkt)
CK-MB
Harnsäure
Hydroxybutyrat-Dehydrogenase
Kreatinin
Natrium
Albumin
Aspartat-Aminotransferase
Chlorid
Gamma-Glutamyltransferase
Harnstoff (Urea)
Kalium
Laktatdehydrogenase
Protein
Alkalische Phosphatase
Bilirubin
Cholesterin
Glukose
HDL
Kreatin-Kinase (CK)
LDL
Triglyceride
It is possible that certain calculated parameters are not specified.
Tests (2)
General Chemistry IV Lyophilized Kit Parameter
Parameter:
Alanin-AminotransferaseAlbuminAlkalische PhosphataseAspartat-AminotransferaseBilirubinBilirubin (direkt)CholesterinGamma-GlutamyltransferaseGlukoseHarnsäureHarnstoff (Urea)HDLKreatininLDLProteinTriglyceride
Probentyp:
PlasmaSerumVollblut
Probenvolumen: 100 µL
Zeit bis zum Ergebnis:420 s
Clinical Emergency Lyophilized Kit Parameter
Parameter:
AmylaseChloridCK-MBGlukoseHarnsäureHarnstoff (Urea)Hydroxybutyrat-DehydrogenaseKaliumKreatin-Kinase (CK)KreatininLaktatdehydrogenaseNatrium
Probentyp:
PlasmaSerumVollblut
Probenvolumen: 100 µL
Zeit bis zum Ergebnis:420 s
General Chemistry IV Lyophilized Kit Parameter
Parameter:
Alanin-AminotransferaseAlbuminAlkalische PhosphataseAspartat-AminotransferaseBilirubinBilirubin (direkt)CholesterinGamma-GlutamyltransferaseGlukoseHarnsäureHarnstoff (Urea)HDLKreatininLDLProteinTriglyceride
Probentyp:
PlasmaSerumVollblut
Probenvolumen: 100 µL
Zeit bis zum Ergebnis:420 s
Clinical Emergency Lyophilized Kit Parameter
Parameter:
AmylaseChloridCK-MBGlukoseHarnsäureHarnstoff (Urea)Hydroxybutyrat-DehydrogenaseKaliumKreatin-Kinase (CK)KreatininLaktatdehydrogenaseNatrium
Probentyp:
PlasmaSerumVollblut
Probenvolumen: 100 µL
Zeit bis zum Ergebnis:420 s
Studies & Product Documents
Related Products
Disclaimer: Diagnoodle is intended solely for healthcare professionals and serves as a guide to in-vitro diagnostics (IVD), particularly point-of-care devices. Product availability and approval may vary regionally. Despite careful research, we assume no liability for the completeness, accuracy, or timeliness of the information provided. All liability is excluded.